Rochelle Thuynsma, Head of Products: Technical
Calcium and silica are fundamental elements in plant and soil systems, each contributing uniquely to cell wall stability, stress mitigation and nutrient dynamics, making their combined role increasingly relevant in the development of sustainable agricultural practices.
CALCINATOR™
CALCINATOR™ is a pectin complexed foliar calcium (Ca) source formulated to enhance the uptake of Ca as well as target Ca to the cell wall. Calcium not only acts as a cell wall and membrane stabiliser, but also as a secondary messenger in a number of developmental and physiological processes. Selected organic compounds increase growth rates, photosynthesis and tolerance against abiotic stress conditions. Stronger cell walls are also associated with increased disease resistance, improved skin quality and a longer shelf life.
The role of Ca
Calcium plays a vital role in plant physiology, particularly in maintaining cell wall structure, membrane integrity and stress tolerance. In fruit crops, insufficient Ca during early fruit development can result in disorders such as bitter pit in apples, blossom-end rot in tomatoes and creasing in citrus. (1) Despite sufficient soil Ca-levels, fruits, especially young fruitlets, often suffer from Ca-deficiency due to restricted translocation and uptake during early stages of development.
Calcium uptake in young fruitlets
Calcium is primarily transported via the xylem, driven by transpiration flow. (2) Young fruitlets, however, have low transpiration rates and weak xylem development, making direct uptake from the roots inefficient during early stages. (3) In fact, the xylem functionality in many fruits becomes progressively reduced as the fruit develops, with the phloem playing a dominant role for nutrient transport, though Ca, being relatively immobile in the phloem, is poorly supplied this way. (4)
Cuticular uptake through the fruit surface provides an alternative route. The fruit epidermis, though protected by a cuticle, is permeable to small ions under certain conditions. Foliar application of Ca has been shown to penetrate the fruit surface, especially when formulated with surfactants or organic ligands. (5) The uptake efficiency is significantly influenced by the molecular form of Ca applied.
CALCINATOR™ mode of action
Pectins are naturally occurring polysaccharides found in plant cell walls, particularly rich in galacturonic acid units that can bind divalent cations like Ca. When Ca is complexed with pectin, it forms a neutral or slightly anionic complex that improves leaf and fruit surface adhesion and facilitates transcuticular movement. (6) Additionally, pectin-Ca complexes are believed to enhance Ca-mobility within the apoplast and possibly the symplast, addressing the traditionally poor mobility of ionic Ca.
These formulations may also act as mimics of the natural Ca-binding processes in the cell
wall, allowing for better integration into developing tissues. This is particularly advantageous in fruitlets, where cell division and wall expansion require constant Ca-availability to maintain pectin cross-linking and tissue firmness. (7)
Enhanced cell wall structure during development can improve post-harvest disease resistance when using CALCINATOR™ (Figure 1).
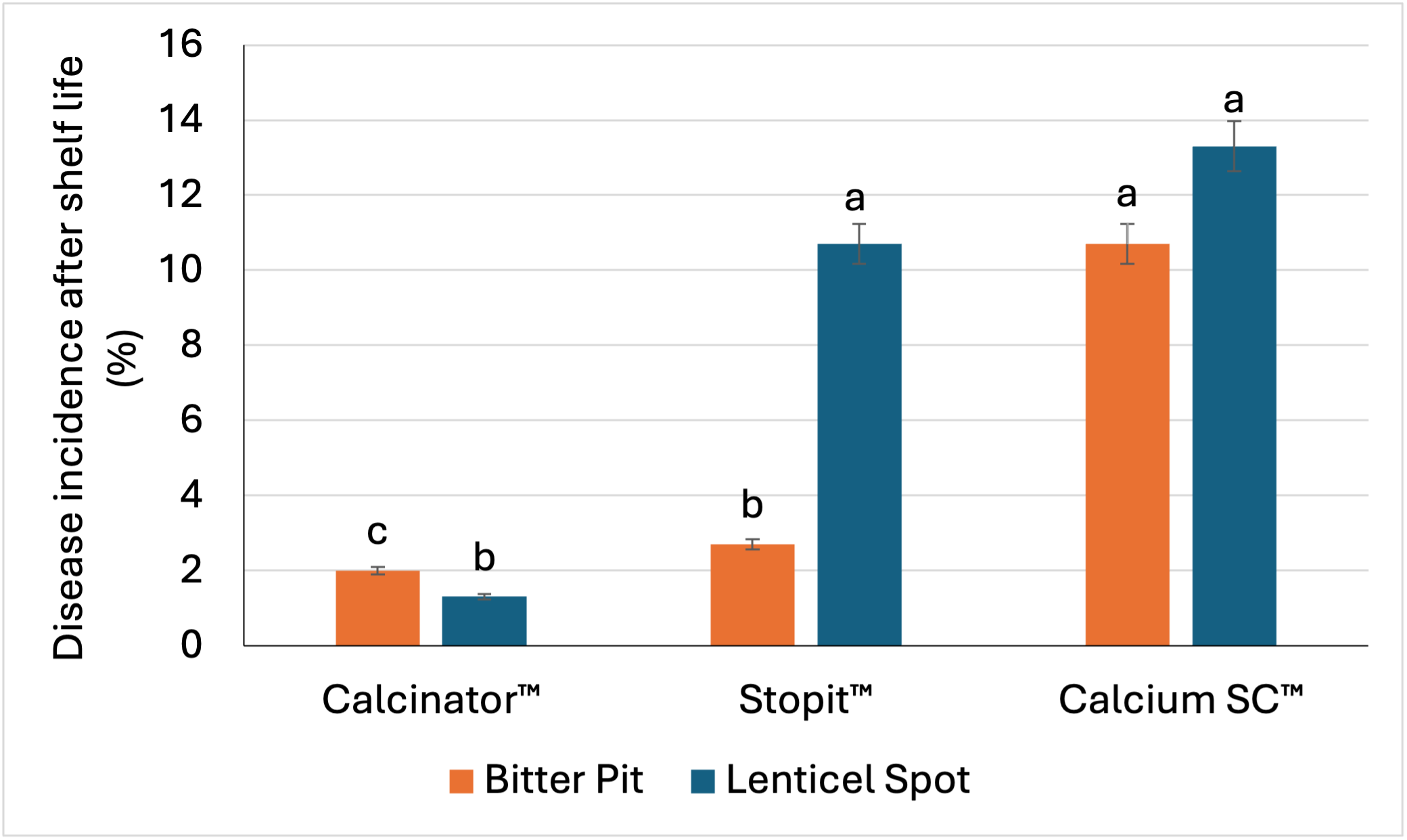

Figure 1: Comparison of post-harvest disease incidence on Golden Delicious apples after simulated shelf life, recorded after foliar applications of CALCINATOR™, CaCl2 and CaCO3 products.
Benefits of CALCINATOR™
From the literature, field trials have shown that foliar applications of pectin-complexed Ca during early fruit development can significantly improve fruit quality parameters and reduce physiological disorders.
In apples, early-season sprays with Ca-pectate formulations reduced bitter pit incidence by up to 60% compared to untreated controls. (8) In citrus, foliar Ca improved peel strength and reduced creasing when applied during the cell division phase. (9) In tomato, blossom-end rot incidence was reduced with foliar Ca applied during fruit set, with improved uptake efficiency using pectin-based formulations. (4)
CALCINATOR™ and KELPURA™
Combining CALCINATOR™ with KELPURA™ further enhances the uptake of Ca and also results in the added benefits of enhancing cell division as well as alleviation of stress from environmental factors (Figure 2).
KELPURA™ is a pure kelp extract derived from Ecklonia maxima containing high levels of salicylic acid which are crucial to work against excessive ABA and ethylene production during flowering and fruit formation. Salicylic acid enhances the uptake of Ca. High levels of benzoic acid further support the plant’s natural metabolism to produce cytokinins as well as salicylic acid, further reducing the influence of environmental conditions on flower and fruit set.
Characterised phytohormones in KELPURA™, such as auxins, cytokinins and brassinosteroids, aid the movement of Ca within the plant, targeting Ca to the cell wall and cell membrane, as well as enhancing uptake by influencing Ca transporter gene expression.
Amino acids in KELPURA™ support Ca-uptake due to their natural complexing properties and include glutamate, glycine, histidine and lysine. Several amino acids in KELPURA™ also aid in the assimilation of Ca. Glutamate and aspartate aid in the movement of Ca within the plant, while serine is involved in cell wall synthesis, directly targeting Ca to the cell wall.
Pectin-complexed Ca is particularly effective when applied at early stages (fruitlets are less than 15 mm in diameter) when cell division and wall construction are most active and demand for Ca is at its peak. (10) The complexed form enhances the Ca-gradient toward fruit epidermal cells, which often act as the first line of defence against Ca-related disorders.

Figure 2: Total Ca in leaf tissue after application of CALCINATOR™ and a combined application of CALCINATOR™ and KELPURA™.
STRENGTH™
STRENGTH™ is a unique source of Ca, boron (B) and orthosilicic acid, blended with natural chelating and organic compounds. STRENGTH™ is a source of orthosilicic acid, the only biologically available source of silicon for plants.
Silicon (Si) is an important, beneficial element required for the proper functioning of cultivated crops. The orthosilicic acid present in STRENGTH™ is rapidly absorbed by the plant, making it an ideal carrier for other plant nutrients, stimulating development and cell growth.
Young fruitlets undergo intensive cell division, wall formation and vascular development, all of which require precise mineral nutrition. Boron is essential for pectin cross-linking and membrane stability, while Si, though not considered essential, plays a critical role in strengthening epidermal tissues and reducing abiotic and biotic stress. (11, 12)
However, the mobility and uptake of both elements, particularly Si, can be limited under field conditions, especially during early fruit stages when root uptake is compromised. Foliar application of orthosilicic acid, a soluble and plant-available form of Si, in combination with B, offers a promising approach to addressing these limitations.
STRENGTH™ mode of action
Silicon is primarily taken up from the soil in the form of orthosilicic acid (H4SiO4), but fruitlets, particularly in their early stages, often receive minimal Si via root-to-fruit translocation due to
low transpiration and limited xylem activity. (13) Furthermore, Si is deposited primarily through the apoplastic pathway, and its phloem mobility is limited. (14)
Foliar-applied Si, particularly as orthosilicic acid, can be absorbed through the cuticle and stomata of leaves and fruit surfaces. Studies show that young fruitlet epidermal cells can absorb Si from foliar sprays, especially when formulated with stabilisers or complexing agents to keep orthosilicic acid in its monomeric, bioavailable form. (15) Once absorbed, Si is polymerised and deposited in the cell walls and subcuticular spaces, strengthening tissues and enhancing mechanical resistance. (16)
Comparing orthosilicic acid and potassium silicate
Traditional foliar Si-formulations often use potassium silicate (K4SiO4), which is alkaline and forms insoluble silica gels when pH drops below 9 (Table 1). This limits its bioavailability, especially on the leaf or fruit surface. (17)
In contrast, orthosilicic acid (H4SiO4) is a neutral molecule (pKa ~9,5) and remains soluble at typical spray solution pH levels (5,5 to 7,0), allowing efficient penetration and assimilation (Table 1).
Orthosilicic acid is also less prone to polymerisation and precipitation on the leaf surface, allowing it to remain in a form plants can use. (12)

Table 1: Comparing orthosilicic acid and potassium silicate
Boron and silicon synergy in fruitlets
Boron is crucial during early fruitlet development for cell wall cross-linking through the formation of borate diesters with rhamnogalacturonan-II (RG-II) pectins. (18) Boron deficiency during this stage can lead to fruit deformation, cracking and abortion.
The combination of B with Si provides synergistic benefits:
• Boron facilitates cell wall elasticity and plasma membrane integrity, key during rapid expansion. (2)
• Silicon enhances cell wall rigidity and reduces water loss, aiding fruitlet resilience under heat or drought stress. (19)
• Together, they optimise the cell wall matrix, balancing flexibility and strength.
Recent studies indicate that foliar applications of orthosilicic acid improve fruit set, reduce fruit drop and enhance skin quality in crops such as apple, citrus and tomato. (20)
Benefits of STRENGTH™
Several trials from the literature have demonstrated the efficacy of orthosilicic acid, Ca and B in supporting young fruitlets. In citrus, the unique STRENGTH™ source of Ca, B and orthosilicic acid applications at fruit set, reduced microcracking and improved peel elasticity. (21) Apple fruitlets treated with STRENGTH™ showed higher Ca-retention and reduced bitter pit, possibly due to reduced epidermal transpiration and stronger cell wall binding. (22) In tomatoes, STRENGTH™ reduced fruitlet abortion under high temperature stress by 35% compared to untreated controls. (23)
These benefits are particularly pronounced when STRENGTH™ is applied in the first 3 to 4 weeks after fruit set.
CONCLUSION
CALCINATOR™ is a pectin complexed Ca source that offers a promising solution to address Ca-deficiency in young fruitlets. By enhancing Ca-availability and uptake through the fruit surface, these formulations support early fruit development, improve structural integrity and reduce physiological disorders. Their mode of action aligns well with the unique challenges of Ca transport in fruitlets, making them an essential component of integrated fruit nutrition programs.
STRENGTH™ is an orthosilicic acid, calcium and boron fertiliser that represents a scientifically validated strategy to enhance young fruitlet development. Compared to silicate salts, orthosilicic acid offers superior stability, foliar uptake and compatibility.
In synergy with B, it supports early structural development, improves fruit quality, and reduces abiotic stress susceptibility. Integration of orthosilicic acid foliar treatments into early-season fertility programmes offers a practical approach to improving yield and fruit resilience.
KELPURA™ is a concentrated liquid biostimulant made from Ecklonia maxima kelp using a proprietary extraction process to preserve its natural bioactives. KELPURA™ supports flowering and fruit set by reducing stress-related hormones and enhancing calcium uptake and movement within the plant. Rich in salicylic and benzoic acids, phytohormones, and amino acids, KELPURA™ improves calcium assimilation and targets it to the cell wall, supporting reproductive development and fruit quality.
CALCINATOR™: Reg. no. B5022.
STRENGTH™: Reg. no. B6635.
KELPURA™: Reg. no. M442 Fertiliser Group 2 | Act 36 of 1947
References:
1. White, P.J., Broadley, M.R. 2003. Calcium in plants. Annals of Botany 92(4):487–511.
2. Marschner, P. 2012. Marschner’s Mineral Nutrition of Higher Plants (3rd ed.). Academic Press.
3. Saure, M.C. 2005. Calcium translocation to fleshy fruit: its mechanism and endogenous control.
Scientia Horticulturae 105(1):65–89.
4. Ho, L.C., White, P.J. 2005. A cellular hypothesis for the induction of blossom-end rot in tomato fruit. Annals of Botany 95(4):571–581.
5. Schlegel, T.K., Schönherr, J. 2002. Penetration of calcium chloride into petal cells of Impatiens walleriana L. Journal of Plant Nutrition and Soil Science 165(4):468–474.
6. Mayer, F.K., Mühling, K.H., Gessler, A. 2017. Foliar application of calcium: An overview of recent advances and future perspectives. Journal of Plant Nutrition and Soil Science 180(6):693–703.
7. Demarty, M., Morvan, C., Thellier, M. 1984. Calcium and the cell wall. Plant, Cell & Environment 7(6):441–448.
8. Ferguson, I.B., Watkins, C.B. 1992. Bitter pit in apple fruit. Horticultural Reviews 13:289–355.
9. Barry, G.H., Castle, W.S., Davies, F.S. 2000. Rootstock and cultivar affect leaf and fruit mineral concentrations of Florida oranges. Journal of the American Society for Horticultural Science 125(2):169–175.
10. Kirkby, E.A., Pilbeam, D.J. 1984. Calcium as a plant nutrient. Plant, Cell and Environment 7:397–405.
11. Epstein, E. 1999. Silicon. Annual Review of Plant Biology 50:641–664.
12. Liang, Y., Sun, W., Zhu, Y.G., Christie, P. 2007. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environmental Pollution 147(2):422–428.
13. Ma, J.F., Tamai, K., Yamaji, N., Mitani, N., Konishi, S., Katsuhara, M., Yano, M. 2001. A silicon transporter in rice. Nature 440:688–691.
14. Raven, J.A. 2001. Silicon transport at the cell and tissue level. In: Silicon in Agriculture. Elsevier, pp. 41–55.
15. Suriyaprabha, R., Karunakaran, G., Yuvakkumar, R., Prabu, P., Rajendran, V., Kannan, N. 2012. Application of silica nanoparticles in maize to enhance growth and yield. Plant Physiology and Biochemistry 51:223–229.
16. Richmond, K.E., Sussman, M. 2003. Got silicon? The non-essential beneficial plant nutrient. Current Opinion in Plant Biology 6(3):268–272.
17. Debona, D., Rodrigues, F.Á., Datnoff, L.E. 2017. Silicon’s role in plant stress responses. Frontiers in Plant Science 8:510.
18. O’Neill, M.A., Ishii, T., Albersheim, P., Darvill, A.G. 2004. Rhamnogalacturonan II: Structure and function of a borate cross-linked cell wall pectic polysaccharide. Annual Review of Plant Biology 55:109–139.
19. Guntzer, F., Keller, C., Meunier, J.D. 2012. Benefits of plant silicon for crops: A review. Agronomy for Sustainable Development 32(1):201–213.
20. Savvas, D., Ntatsi, G. 2015. Biostimulant action of silicon in horticulture. Scientia Horticulturae 196:66–81.
21. Alvarez, R., Rodríguez, P., Muñoz, D. 2020. Use of silicon and boron to reduce microcracking in citrus peel. Scientia Horticulturae 261:108980.
22. Fernández, V., Sotiropoulos, T., Brown, P.H. 2013. Foliar Fertilization: Scientific Principles and Field Practices. International Fertilizer Industry Association.
23. Samuels, A.L., Glass, A.D.M., Ehret, D.L., Menzies, J.G. 2021. Effect of foliar orthosilicic acid and boron on heat stress tolerance in tomato fruitlets. Plant Physiology Reports 26(2):217–225.

